Differential Digital Confocal Aperture
Why Line-scanning Confocal Imaging?
Probe-based fluorescence microendoscopy has demonstrated great potential to improve early cancer detection. When imaging thick, highly scattering biological tissue, the high level of scattering usually necessitates suppressing unwanted out-of-focus signal to obtain images with good contrast. Line-scanning confocal imaging is an appealing technique for clinical translation, since it allows for video-speed frame rate at relatively low complexity and cost.
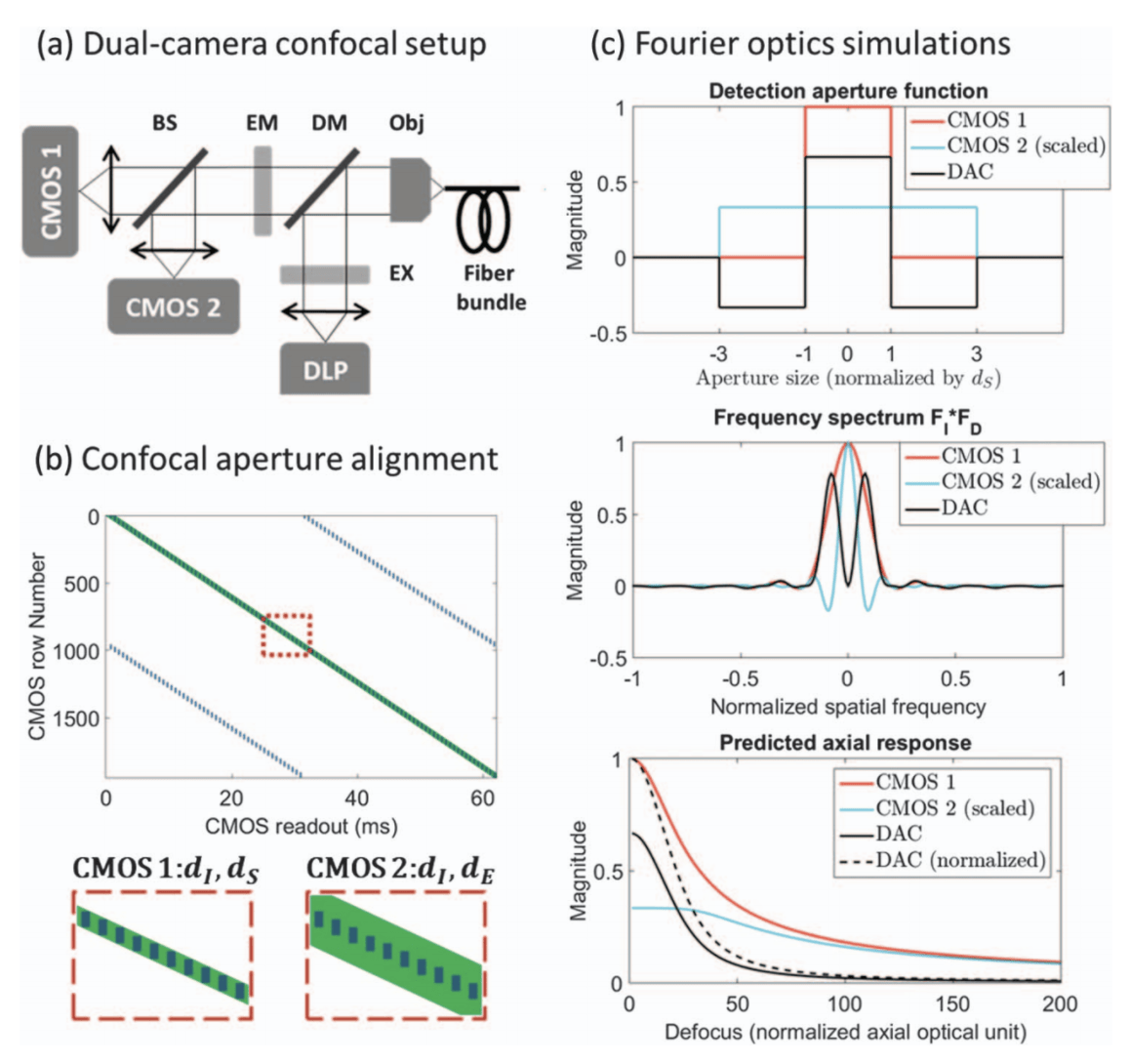
Simple differential digital confocal aperture to improve axial response of line-scanning confocal microendoscopes
Differential Aperture Confocal (DAC)
More Info
DAC HRME is a dual-camera line-scanning fluorescence microscope. Blue light from a LightCrafter 4500 DLP (Texas Instruments; total power is 1.3 mW at the
sample end in each confocal scanning cycle) is collimated by a lens (f 125 mm) that replaces the built-in DLP lens to provide fluorescence excitation. Two 8-bit CMOS sensors (Mako U503, Allied Vision) were used for fluorescence descanning in rolling shutter mode. The microscope is coupled with a coherent fiber bundle [FIGH-30-850N, Myriad Fiber Imaging; 790 μm circular field of view (FOV)] as an image relay, and collected fluorescence is divided by a beam splitter.
Low-cost
More Info
Line-scanning confocal imaging allows for video-speed frame rate at relatively low complexity and cost.
Line-scanning confocal microendoscopy offers video-rate cellular imaging of scattering tissue with relatively simple hardware, but its axial response is inferior to that of point-scanning systems. Based on Fourier optics theory, we designed differential confocal apertures with a simple subtraction technique to improve the line-scanning sectioning performance. Taking advantage of digital slit apertures on a digital light projector and a CMOS rolling shutter, we demonstrate real-time optical sectioning performance comparable to point scanning in a dual-camera microendoscope (<$6, 000). We validate the background rejection capability when imaging porcine columnar epithelium stained with fluorescent contrast agents with different uptake mechanisms and staining properties.
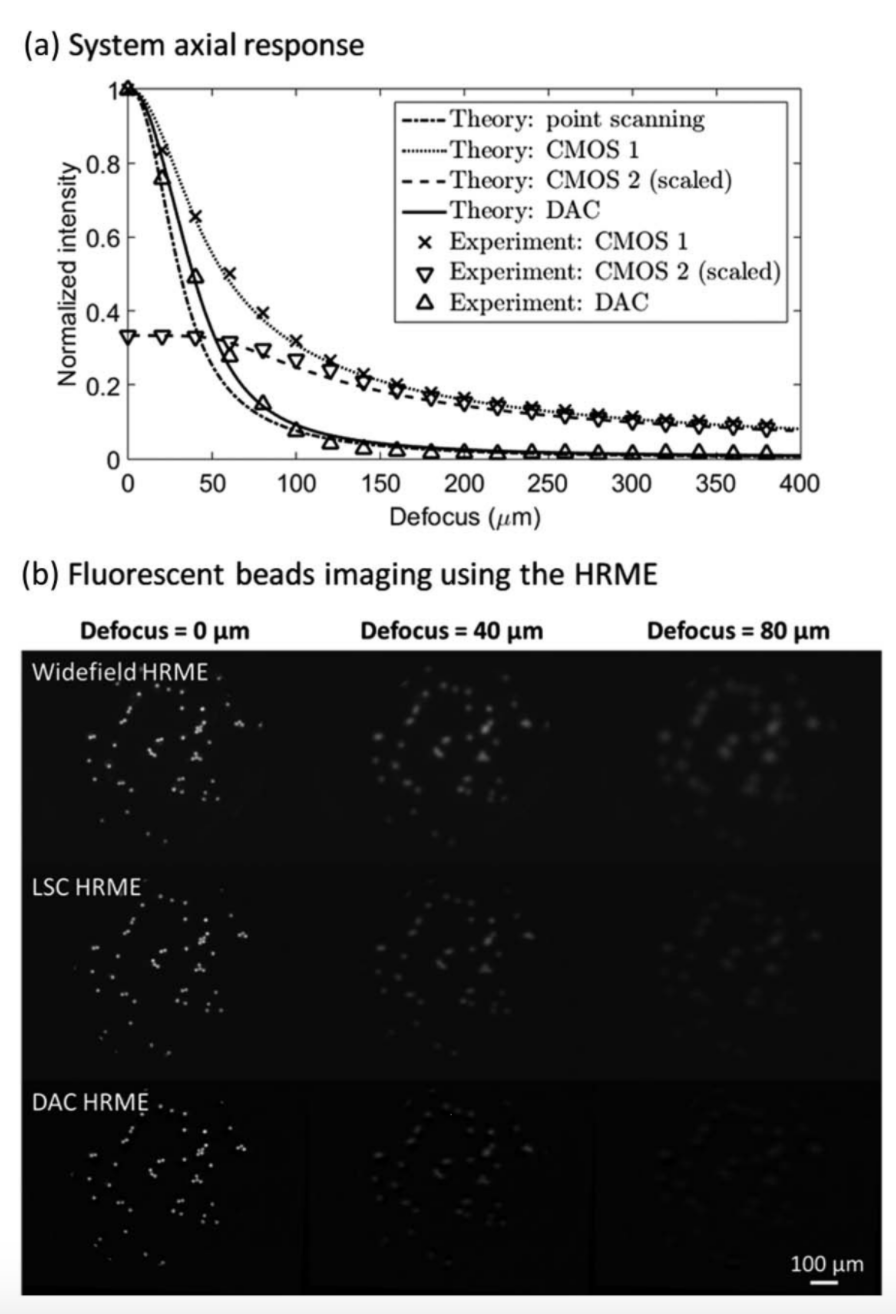
(a) Axial responses of different imaging configurations (theory and experiment), and (b) fluorescent beads imaging using the HRME.
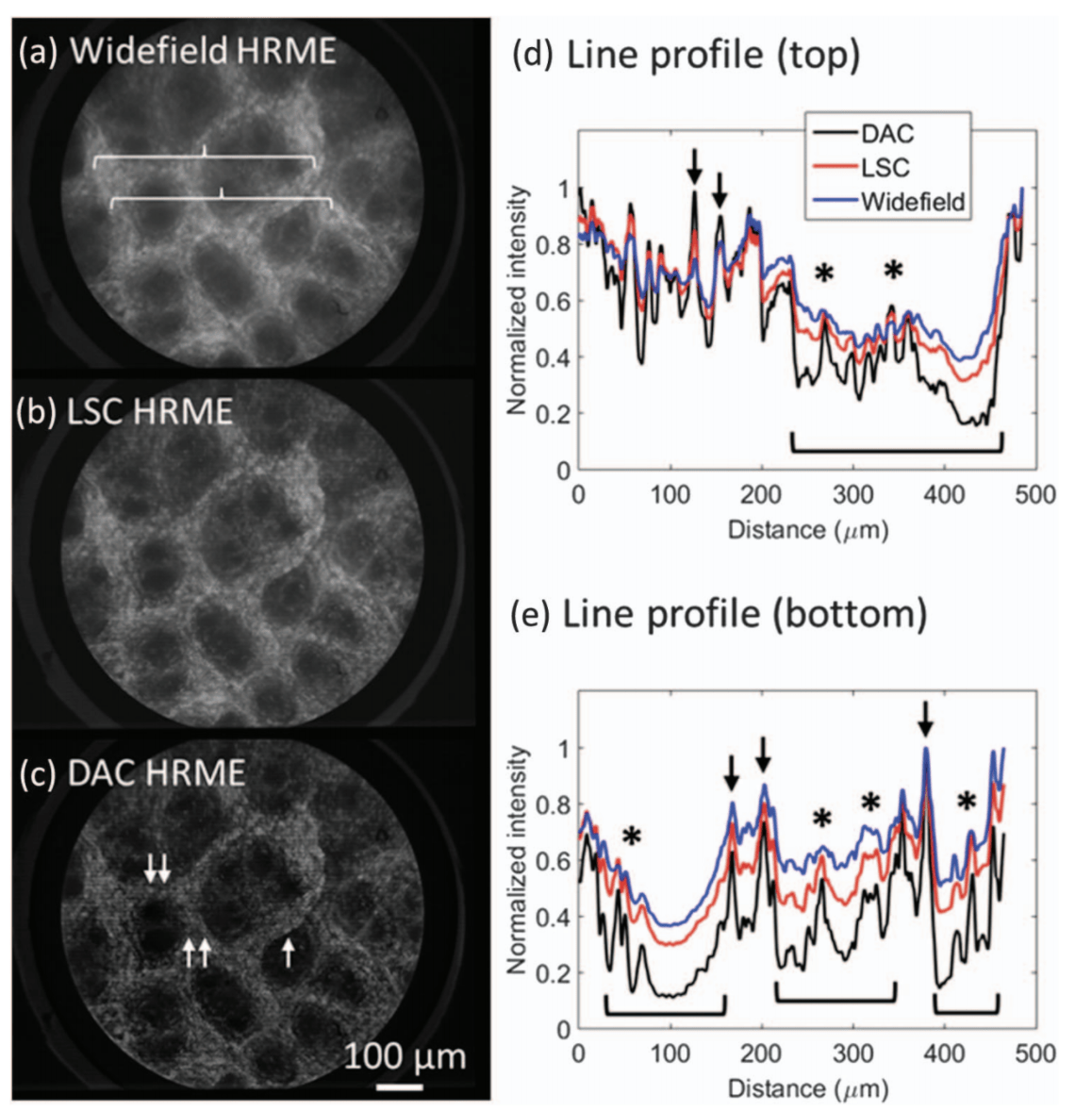
Ex vivo imaging of gastric columnar epithelium stained with
proflavine. Two lines indicated by braces in (a) are profiled in (d) and
(e), highlighting enhanced contrast of nuclei (arrows) on the superficial
lining and glandular structures (asterisks) within the pits (brackets) in
the DAC image.
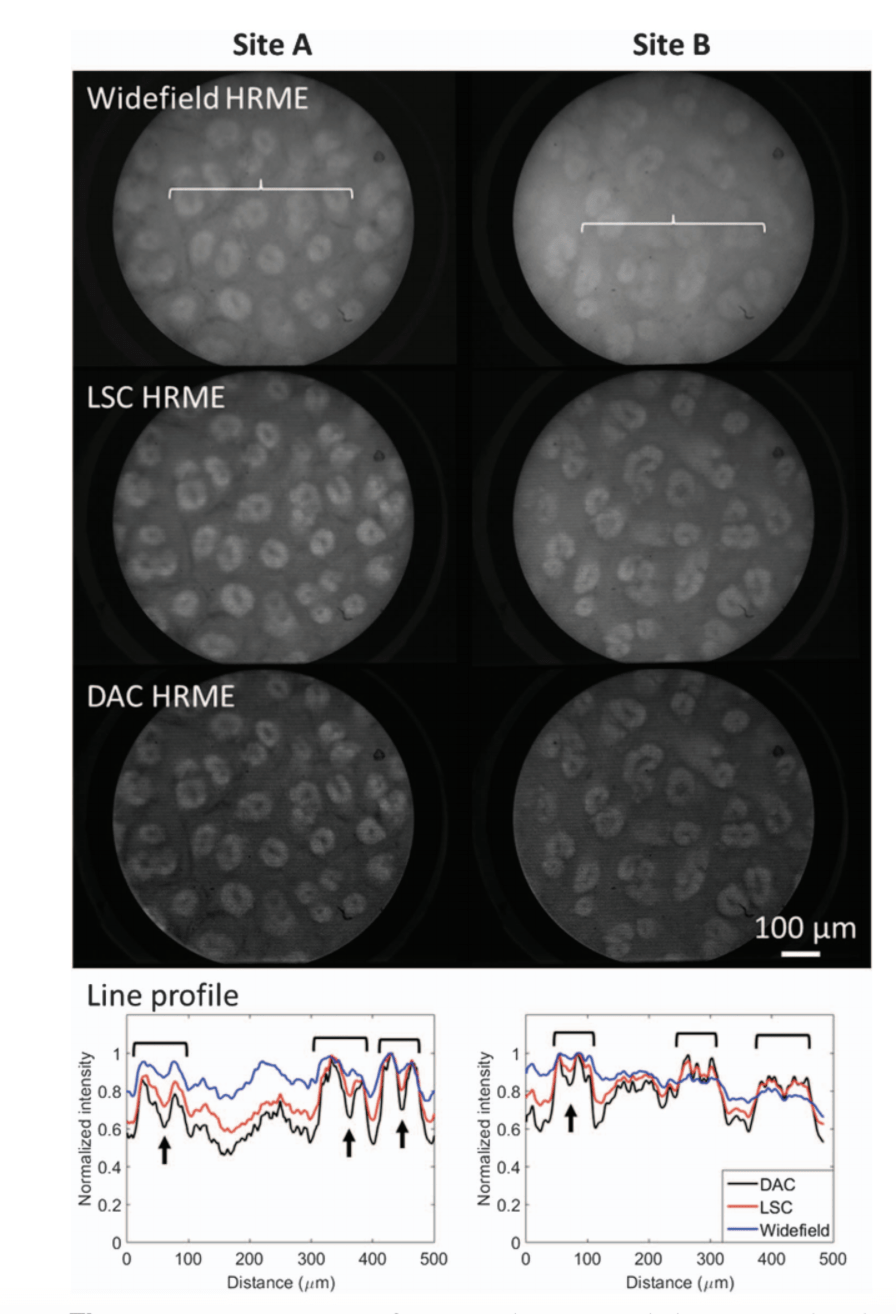
Ex vivo imaging of gastric columnar epithelium stained with fluorescein. Two lines indicated by the braces in the widefield images are quantified, showing enhanced contrast of pits (brackets) and lower signal levels due to elevated glands (arrows).
People

Yubo Tang

Alex Kortum
Imran Vohra
Jennifer Carns
Sharmila Anandasabapathy

Rebecca Richards-Kortum
