Confocal microendoscope

Cervical cancer incidence and mortality rates remain high in medically underserved areas. In this study, we present a low-cost (<$5,000), portable and user-friendly confocal microendoscope, and we report on its clinical use to image precancerous lesions in the cervix. Imaging performance is evaluated in ex vivo and in vivo pilot studies; results demonstrate that the confocal microendoscope can enhance visualization of nuclear morphology, contributing to significantly improved recognition of clinically important features for detection of cervical precancer.
In vivo imaging of cervical precancer using a low-cost and easy-to-use confocal microendoscope
In this study, we optimize the confocal microendoscope to improve its portability and clinical ease-of-use. To facilitate technology dissemination, we develop an automated algorithm for fine alignment of confocal apertures that can be easily misaligned during device transportation. The automated and fast (< 2 mins) calibration procedure tunes confocality at the micron level and ensures high quality confocal imaging, which would otherwise require manual alignment of mechanical parts by a trained operator. As real-time imaging is displayed to the clinicians in confocal mode, we save two sequential frames in widefield (non-confocal) and confocal modes when image acquisition is triggered using a foot pedal. The sequential image acquisition in two modes allows us to evaluate contrast improvement due to confocal background rejection with clinical data in identical fields of view (FOVs) during ex vivo imaging, and similar FOVs during in vivo imaging as the fiber is hand-held.
Optical Design & Mechanical Assembly
More Info
Optical and mechanical assembly of the confocal microendoscope (A) in a compact enclosure, which allows for convenient deployment for in vivo imaging of cervical lesions in a mobile clinic in the Rio Grande Valley, Texas (B). Detailed aperture design and spatiotemporal alignment in confocal and widefield (non-confocal) modes is shown in Fig. C. DLP: digital light projector; DMD: digital micromirror device; EM: emission filter; EX: excitation filter; DM: dichroic mirror.

Automated Confocal Aperture Alignment
More Info
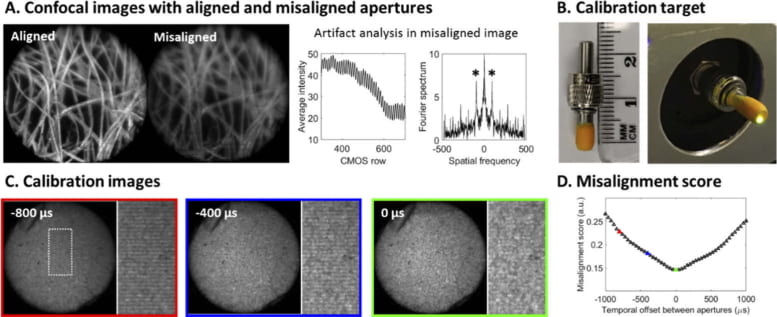
Automated alignment of digital confocal apertures using a simple calibration target. (A) Confocal aperture misalignment compromises background rejection and causes scanning artifacts when imaging lens paper colored with a fluorescent highlighter. (B) A simple calibration target provides a stationary scene to facilitate aperture alignment. (C) Images of the calibration target are acquired with varied temporal offsets by adding a delay between two confocal aperture sequences. (D) An alignment score is calculated based on the calibration images to evaluate the magnitude of scanning artifacts as a function of aperture temporal offset. Circular FOV: 790 µm.

Ex vivo imaging of LEEP specimens using the microendoscope in widefield and confocal modes. The confocal microendoscope better resolves various clinically important features. These findings are confirmed in corresponding profiles of line scans (white brackets in images), showing enhanced contrast of nuclei (asterisks), glandular patterns (black brackets in line profiles) and vascular network (white arrows in confocal image of site C and black arrows in corresponding line profile). GC: global contrast. Circular FOV: 790 µm.
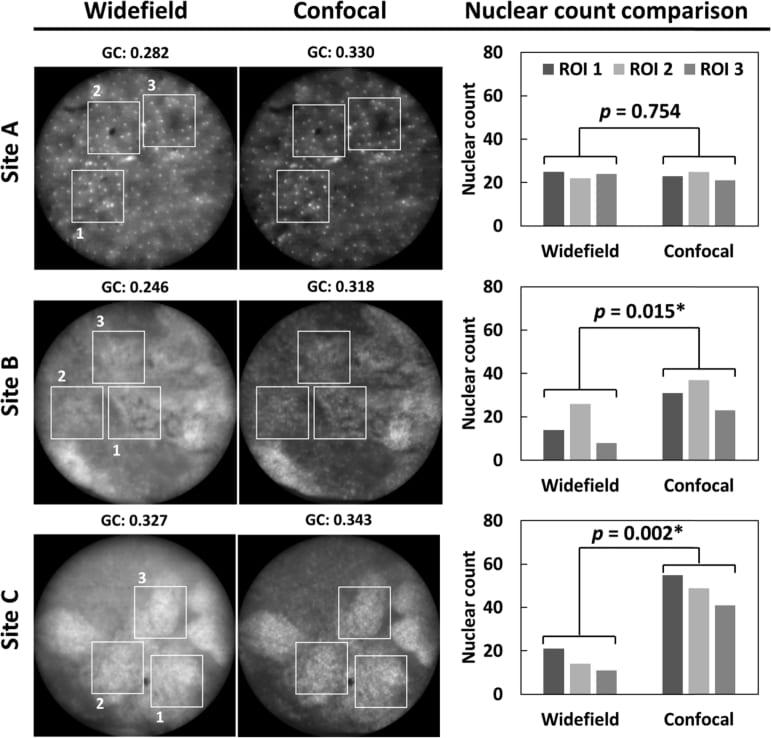
Comparison of nuclear count identified by expert microendoscope users in widefield and confocal microendoscope images of squamous (site A) and columnar (sites B and C) epithelium. Three ROIs are cropped in each site and nuclei that can be individually outlined are counted by three experienced users. The nuclear count is compared for widefield and confocal images using a paired t-test. *: p < 0.05. GC: global contrast. Circular FOV: 790 µm.

In vivo images of benign sites (A – C) and neoplastic lesions (D – E) in the cervix. In each panel, widefield (left) and confocal (right) images are shown with the corresponding histopathology diagnosis provided above each image pair. In vivo confocal imaging reveals morphological alterations associated with cancer progression, which are consistent with histopathology. Compared with widefield mode, the confocal mode better highlights these architecture changes, especially in regions with crowded nuclei and high out-of-focus background. White arrows: glandular involvement. GC: global contrast. Circular FOV: 790 µm.
People

Yubo Tang

Alex Kortum
Sonia G. Parra
Imran Vohra
Andrea Milbourne
Preetha Ramalingam
Paul A. Toscano

Kathleen M. Schmeler

Rebecca R. Richards-Kortum
