Health Sense
Managing Clinical Trials
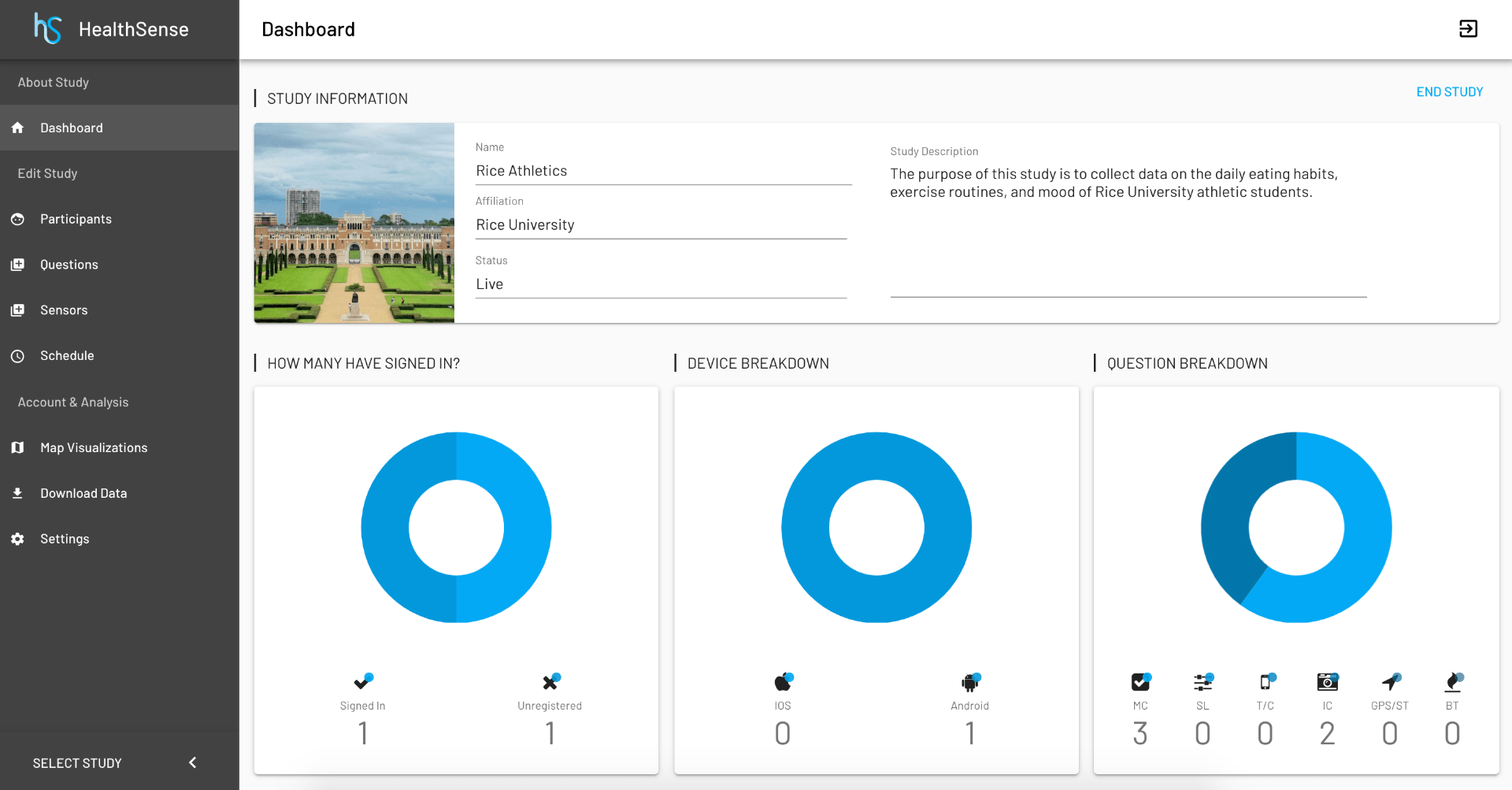
With the rise of ever-more sophisticated wearables and sensing technologies, mobile health continues to be an active area of research. However, from a clinical researcher’s point of view, testing novel use of mobile health innovations remains a major hurdle, as composing a clinical trial using a combination of technologies still remains in the realm of computer scientists. We take a software-inspired viewpoint of clinical trial designs to design, develop and validate HealthSense to enable expressibility of complex ideas, composability with diverse devices and services while maximally maintaining simplicity for a clinical research user.
Study State Manager
A key innovation in HealthSense is the concept of a study state manager (SSM) that modifies parameters of the study over time as data accumulates and can trigger external events that affect the participant; this design allows us to implement nearly arbitrary clinical trial designs. The SSM can funnel data streams to custom or third-party cloud processing pipelines and the result can be used to give interventions and modify parameters of the study. HealthSense supports both Android and iOS platforms and is secure, scalable and fully operational.
Simplicity
More Info
Our targeted users are clinical researchers, so it is important that common trial designs can be deployed using a simple graphical user interface (GUI).
Composability
More Info
The platform must enable researchers to combine a diverse set of data streams, e.g. external sensors, with processing pipelines that can interactively extract useful properties of the data, and adapt the study’s behavior as more data comes in.
Expressibility
More Info
The platform needs to have enough flexibility to realize a wide range of trial and intervention designs and act as a test bench for exploring the efficacy of new trial designs.



People

Aidan Curtis
Amruta Pai
Jian Cao
Nidal Moukaddam

Ashutosh Sabharwal
